|
|
|
|
Tannins revisited - changing perceptions of their effects on animal system Singh B*, Bhat T. K. Regional Station Indian Veterinary Research Institute Palampur - 176 061, India *Reprint request: Dr. B. Singh, Fax: +91-1894-33063; E-mail: ivriplp@nde.vsnl.net.in.
Abstract Tannins are a prominent class of compounds which constitute the plant secondary metabolites group. The two major structural groups of tannins are hydrolyzable tannins and condensed tannins. Due to their widespread nature and ability to complex with proteins and other biomolecules, they exert both harmful and beneficial effects on organisms. Hydrolyzable tannins have toxic effect on the animals that feed on forages rich in these tannins. High concentrations of dietary condensed tannins (6–12% DM) depress voluntary feed intake, digestive efficiency and animal productivity. In contrast, forages containing moderate concentrations of condensed tannins (2–4% DM) can exert beneficial effects on protein metabolism in ruminants especially sheep, by slowing degradation of dietary protein to ammonia by rumen microorganisms and increasing protein outflow from the rumen. This results in the increased absorption of amino acids in the small intestine and ultimately leads to increases in lactation, wool growth and live weight gain, without changing voluntary feed intake of the animal. Dietary condensed tannins can also contribute to improved animal health by reducing the detrimental effects of internal parasites in small ruminants and the risk of bloat in cattle. Therefore, forages containing moderate concentrations of condensed tannins can increase sustainability and productivity in intensive grazing systems through increasing the efficiency of animal production, decreasing urinary nitrogen excretion and reducing chemical inputs such as anthelmintics and detergents to control rumen bloat in cattle Top Key words Hydrolyzable tannins, Condensed tannins, Negative effects, Positive effects. Top | Introduction Tannins are water-soluble polyphenolic compounds of varying molecular weight, found as secondary compounds in pteridophytes, gymnosperms and angiosperms including some leguminous plants (Bhat et al. 1998). Based on their structures and properties, they are distributed into two major classes - hydrolysable tannins (HT) and condensed tannins (CT). Hydrolysable tannins are composed of esters of gallic acid (gallotannins) or ellagic acid (ellagitannins) with a sugar core which is usually glucose and are readily hydrolysed by acids or enzymes into monomeric products. Condensed tannins are hydrolytically cleaved to anthocyanidins and related compounds and are more correctly called proanthocyanidins or polyflavanoids. The structure and chemistry of tannins are complex, with variability in their stereochemistry, molecular size and polymeric form producing tannins specific to each plant in which they are found. As tannins are present in large number of feeds and forages, they are one of the most common anti-nutritional factors. These polyphenolic polymers are of relatively high molecular weight and have the capacity to form complexes with carbohydrates and proteins. Low molecular weight CT oligomers are more reactive and have higher protein precipitating capacities than high molecular weight polymeric tannins (Butler and Rogler, 1992). Formation of complexes of tannins with nutrients, especially proteins, has both negative and positive effects on their utilisation (Reed, 1995). |
Tannins, especially HT, when present in plants can, in general, adversely affect herbivore nutrition by reducing intake, protein digestibility, inhibiting digestive enzymes or by direct systemic toxicity (Kumar and Singh, 1984). This leads to a reduction in their feed intake, adversely affects rumen fermentation and significantly depresses digestibility of almost all the nutrients. HT are toxic and cause poisoning in animals if sufficiently large amounts of tannin-containing plant material, such as leaves of oak (Quercus spp.) and yellow-wood (Terminalia oblongata) are consumed. (Garg et al. 1992; Fillipich et al. 1991). Tannins inhibit the activity of enzymes of rumen microbes (McLeod, 1974; Martin and Akin 1988; Makkar et al. 1988; Bae et al. 1993). CT in high concentrations have been shown to inhibit extracellular endoglucanase activity of Fibrobactersuccinogenes (Bae et al. 1993) and extracts of condensed tannins from Onobrychis viciifolia reduced growth and proteolytic activity of Butyrivibrio fibrisolvens, Ruminobacter amylophilus and Streptococcus bovis (Jones et al. 1994). |
The reviews that have appeared in the past on effect of tannins on ruminant nutrition have laid stress on their anti-nutritional nature (McLeod, 1974; Kumar and Singh, 1984; Makkar et al. 1987; Mangan, 1988; Kumar and Vaithiyanathan, 1990). Increasing attention has recently been given to CT as there is a growing realisation about their role in human and animal well-being. A lot of work has been published on the positive effects of feeding tanniniferous forages having low levels CT to ruminants (Reed, 1995; Lowry et al. 1996; Barry and McNabb 1999). These findings warrant a fresh look at the present scenario on effects of tannin feeding on the nutritional status of ruminants. This review focuses on the emerging area of the beneficial effects of condensed tannins at low to moderate level of intake on ruminant productivity. |
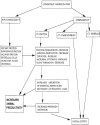
Chemical structure of condensed tannins Condensed tannins or proanthocyanidins are derived from the polymerization of flavan-3-ol-units, and they normally occur in plant vacuoles. The flavan-3-01 monomer units can be linked by C4/C9 or C4/C6 interflavanoid linkages which effects the shape of the CT polymer chain (Ferreira et al. 1999). The number of monomeric units can vary and this determines the degree of polymerization from bi-, tri- and tetraflavanoids to higher oligomers. These can then produce an infinite variety of chemical structures which in turn effect the biological properties of the CT. Effects of condensed tannins on voluntary feed intake, digestion process and utilization efficiency of digested nutrients The positive effects of CT are depicted in Figure 1. CT are secondary plant compounds that can bind with protein by hydrogen bonding at near neutral pH (pH 4.0–7.0) to form CT-protein complexes, but dissociate and release protein at pH <3.5 (Jones and Mangan 1977). They are released during mastication, bind with feed and salivary proteins forming insoluble complexes at rumen pH and are released from the complex in the abomasum. Much work has been conducted upon the effects of CT on nutrient supply of sheep fed fresh forage diets, using controlled indoor experiments with individually fed animals. Barry and Duncan (1984) found a substantial depression of 27% in the voluntary feed intake (VFI) of sheep which grazed on Lotus pendunculatus having high dry matter CT concentrations (6.3–10.6 %). Smaller depressions of 12% in VFI were produced by 5.5% CT in Lotus pendunculatus (Waghorn et al 1994). However, medium CT concentrations in sulla (4.5%) and in Lotus corniculatus (3.4 - 4.4 %) had no effect upon VFI (Terrill et al. 1992; Wang et al. 1996a, Wang et al. 1996b).
Duodenal non-amino nitrogen (NAN) flow can be used as an index of protein nitrogen leaving the rumen. With different cultivars of ryegrass (Lolium perenne) and white clover (Trifoliurn repens), which contain only traces of CT, duodenal NAN flow is only about 0.75 of nitrogen intake, illustrating the extensive absorption of ammonia from the rumen. However; with Lotus spp. duodenal NAN flow increased linearly with increasing CT content and equalled nitrogen intake at ∼ 4% CT concentration. McNabb, et al. (1996) attributed this effect to the action of CT in Lotus spp. in slowing the rates of both solubilization and degradation of forage proteins by rumen microbes, especially that of the principal leaf protein, ribulose-biphosphate carboxylase/oxygenase (Rubisco). Waghorn, et al. (1987, 1994), found a difference due to CT level on the apparent absorption of essential amino acids (EAA) from the small intestine of sheep fed on fresh L. corniculatus (2.2% CT) and L. pedunculatus (5.5% CT). The CT of L. corniculatus increased both abomasal flow and the net absorption of EAA from the small intestine (53 and 59% respectively), with no effect on apparent digestibility in the small intestine, while CT of L. pedunculatus increased abomasal flow by 30% in sheep fed on this forage. However, this was counteracted by reduced apparent digestibility in the small intestine, with there being only a 10% increase in apparent absorption of EAA from the small intestine. These findings with L. pedunculatus could be due to differences in CT concentration with L. corniculatus and the different reactivity of CT of the two species with plant proteins due to dissimilarities in their CT structure (Foo et al. 1996, Foo et al. 1997). McNabb, et al. (1998) were of the opinion that the differences in CT structure between L. corniculatus and L. pendunculatus were insuffcient to cause any appreciable difference in the in vitro precipitation of Rubisco when their CT extracts were reacted with total soluble leaf protein from white clover. However, the CT extract from L. pendunculatus was more effective at reducing Rubisco degradation by ruminal microbes than the CT extract from L. corniculatus (Aerts et al. 1999b). These findings suggest that protein precipitation by the CT may be more responsive to the relative molecular mass of the CT, and to a lesser extent, effected by the prodelphinidin content, whereas the effect of CT on the degradation of protein by ruminal microorganisms may be more responsive to difference in the flavan-3-ol composition of the CT. This aspect, besides the effects of the CT in not releasing some amino acids in the small intestine, increasing endogenous protein secretion or inactivating digestive enzymes, requires further investigation. The effects of CT upon rumen fermentation of carbohydrate and protein has been explained by the concept of ‘free tannin’ developed by Barry and Manley (1986), and is defined as the CT not precipitated in high-speed centrifugation of plant mascerates. The plant proteins precipitated 90% of the CT and 10% was free in solution upto a total CT concentration of about 9%, whereas increments in total CT concentration above 9% were all released as ‘free tannin’. Thus for Lotus spp. almost all the CT reacted with proteins in the host plant until the binding capacity of this system had been saturated (at ∼ 9% CT). It has been suggested that insoluble CT functions through reducing plant protein degradation in the rumen, whereas free CT can react with and inactivate microbial enzymes, explaining why high levels of free CT reduce rumen carbohydrate digestion. This concept also explains why mixing CT-containing and non CT-containing temperate forages seldom produces a beneficial outcome (Beever and Siddons, 1986), as the CT will preferably react with proteins in the forage of the CT-containing plant. It also explains why there is limited transfer of CT-induced protein-precipitating activity through rumen fluid from CT-containing to non CT-containing plants. Beneficial effects of forage mixing can only be expected if the content is extremely high and the protein content relatively low in the CT-containing plant, thus releasing some ‘free’ CT to bind with proteins in the non CT-containing plant. These conditions occur with some tropical legume forages and legume shrubs, especially if grown under low soil-fertility conditions, and some advantage may occur if they are added as supplement to a diet of non CT-containing tropical grasses. Wang, et al. (1996a) investigated the action of CT in lactating ewes rearing twin lambs that were grazing L. corniculatus. CT had no effect upon milk secretion in early lactation but increased the secretion rates of whole milk, lactose and protein by 21,12 and 14% respectively, during mid-and late-lactation The diet selected was also of high nutritive value, containing 3.6% N and 4.4% CT, with an organic matter digestibility of 0.73. Milk protein concentration was unaffected by CT, but action of CT reduced milk fat content by ∼ 1%. These results have implications for the dairy industry, as a nutritional treatment which simultaneously increase the efficiency of milk protein production whilst reducing fat content would be very desirable for human nutrition. Experiments of this type with dairy cows were conducted by Woodward et al. (1999) and they found that cows fed on L. corniculatus had higher milk yields than those fed on L. comiculatus+PEG, ryegrass or ryegrass+PEG, indicating that CT contributed to 42% of the increased milk yield that resulted from feeding L. corniculatus rather than ryegrass. CT had no effect on forage intake but accounted for all the increase in herbage conversion efficiency. It also caused a 57% increase in the milk protein of cows fed L. corniculatus when compared with milk from cows fed other forage preparations. In L. corniculatus fed cows, CT caused lowering of milk fat level but there was no effect on lactose content. However, herbage and CT had no effect on the casein or whey protein concentrations. Bhatta, et al. (2000) observed an increase in body weight and milk protein content of cross-bred dairy cows fed 7.5% tamarind seed husk, a tannin-rich agricultural by-product. For studying the effect of CT on ovulation rate, trials were carried out with ewes grazing perennial ryegrasdwhite clover pasture and L. corniculatus, containing 0.1 and 2.3% CT respectively, Mean ovulation rates of ewes fed on L. corniculatus were greater than those of ewes grazing pasture, showing a clear response to CT (Min et al. 1999). It was also seen that action of CT increased lambing percentage through increasing fecundity with no effect on fertility. The emerging role of condensed tannins in organic livestock farming Ruminants grazing forage diets are subject to a number of diseases, some of which have a nutritional component. Two such conditions are rumen frothy bloat in cattle and internal parasitic infections in grazing sheep, cattle, deer and goats. Both are currently controlled by regular oral administration of chemicals - detergents in the former condition to disperse the foam and anthelminitic drenches in the latter case to kill the parasites. These remedies control both conditions in the short term but have long-term problems. First, they treat the symptoms and not the cause. Second, they cause consumer concerns about sustained use of chemicals and possible product residues, leading to longer withholding periods. Finally, in the case of anthelmintics, sustained regular use over many years has led to the development of parasites that are resistant to the control measures. Therefore, there is a need for development of new control measures that are more nutritionally based and ecologically sustainable. Bloat is caused by very high solubility of forage proteins leading to the development of a stable foam in the rumen, and is very prevalent in cattle fed on legumes, especially in spring (Mangan, 1959). Because of their protein-precipitating properties, grazing of CT-containing legumes has long been known to eliminate bloat (Jones et al. 1973). However, the minimum plant CT concentration needed to make forage bloat-safe was not known. This has recently been proposed to be 0.5 % or greater (Li et al. 1996). Most common legumes and grasses used in temperate agriculture have CT concentrations well below this value, and both conventional plant breeding and genetic engineering techniques are now being examined to try and increase these levels (Aerts et al. 1999a). Parasitism of the abomasum and small intestine causes extensive protein losses in sheep (Kimambo et al. 1988), and re-directs protein synthesis away from skeletal muscles and into repair of gut tissues, leading to reduced N retention (MacRae, 1993). Increasing dietary protein intake and abomasal infusion of protein results in the animal being much better, able to tolerate these infections and improves N retention (Brown et al. 1991; Coop and Holmes, 1996), with the main effect of increased protein supply being to increase the rate of acquisition of immunity. Niezen, et al. 1993a, Niezen, et al.1993b, 1994 and 1996 initially observed that sheep fed natural diets rich in CT gained weight more rapidly than controls. Subsequent studies showed that lambs grazing CT-containing forages (L. pedunculatus and Suet al), are better able to tolerate parasitic infections than lambs grazing non CT-containing forage (lucerne) and have both increased growth and lower worm burdens (Niezen et al. 1995, Niezen et al. 1998). Similar observations have been made in deer infected with gastrointestinal parasites and lungworms (Molan et al. 2000a, Molan et al. 2000b; Hoskin et al. 2000). Butter, et al. 1998 and Athanasiadou, et al, 2000 observed a reduction in the burden of Trichostrongylus colubnformis in trickle-infected or single dose-infected sheep which consumed a CT containing extract incorporated in their feed, compared with infected sheep fed a standard ration. A 37–63% inhibition in migration of T. colubriformis larvae has been reported for CT extracts of seven forages (Molan et al. 2000c). The addition of polyethylene glycol (PEG) to the CT extracts eliminated 81–93% of their larval migration inhibition activity. A similar effect of PEG on CT activity has been observed in the case of goats grazing on CT-rich browse (Kabasa et al. 2000; Molan et al. 2000d). The faecal helminth egg counts in these free-ranging goats which was quite low in control, nearly doubled in animals administered PEG orally, thus negating the beneficial effect of CT in lowering the host's gastrointestinal parasitic burden. The above studies suggest that condensed tannins may have an effect on the populations of larval and/or adult worm population of gastrointestinal nematodes in ruminants. The question is whether this effect is a direct anthelmintic effect, or indirect, or a combination of both. An indirect metabolic effect could be mediated through the ability of tannins to bind to dietary protein in the buccal cavity and rumen, and thus increase the amount of undegradable protein passing to the small intestine for absorption (Woodward and Reed, 1997). Such an increase has been shown to improve the ability of ruminants to mount an effective immune response against gastrointestinal parasites (Coop and Kyriazakis, 1999). There may be involvement of two possible mechanisms. First, improved EAA supply from the action of the CT may counteract the protein loss caused by gut parasitism and may stimulate the immune system, and second the CT may directly react with and inactivate parasite larvae during passage through the gut. Forages containing CT may thus lead to the development of nutritionally-based and ecologically-sustainable systems for disease control in grazing animals and allow the reduction in the use of anthelmintic drugs. Top Conclusions and Future Projections The ideal CT concentration for ruminant nutrition has been suggested to be in the range of 2–4 % (Barry 1989; Waghorn et al. 1990). Improved estimation methods have shown the presence of trace amounts of CT (0.1–0.2%) in most of the common grasses and legumes grazed in temperate agriculture. However, results to date show that this is too low to reduce protein solubility and degradation in the rumen, reduce bloat and gastrointestinal parasitism, and a low to moderate level of CT is suggested (Figure 1) i.e. a minimum concentration of 0.5% (for control of bloat) to 2–4% (for control of internal parasites). Robertson, et al. 1995 have proposed a system for controlling internal parasites in sheep with minimal anthelmintic usage in which CT-containing and CT-free plants are alternatively grazed. An agronomic evaluation of tanniniferous plants has also to be done as this will determine how they can be incorporated into ruminant production systems. By integrating CT-containing forages into intensive grazing systems in this way, a much more sustainable control of both internal parasites and bloat, with reduced dependence on chemicals and simultaneous increase in production efficiency of animals will be achieved. Evaluation of traditional plant breeding methods and genetic engineering techniques and their application for increasing CT concentration in common legumes such as white clover, red clover (Trifolium pratense) and lucerne offers exciting future possibilities. The effects of long term feeding of CT-containing forages on animals and their rumen microbial ecosystem also need to be investigated. |
Top Figure | Figure 1: Positive effects of condensed tannins at low to moderate levels
|  | |
|
| References | | AertsR. J., BarryT. N., McNabbW. C.
1999a.
Polyphenols and agriculture: beneficiaI effects of proanthocyanidins in forages.
Agriculture, Ecosystems and Environment,
75:
1–12. TopBack | | AertsR. J., McNabbW. C., MolanA., BrandA., BarryT. N., PetersJ. S.
1999b.
Condensed tannins from Lotus corniculatus and Lotus pedunculatus exert different effects on the in vitro rumen degradation of ribulose-l,5-biphosphate/carboxylase (Rubisco) protein.
Journal of the Science of Food and Agriculture,
79:
79–85. TopBack | | AthanasiadouS., KyriazakisI., JacksonF., CoopR.L.
2000.
Effects of short term exposure to condensed tannins on adult Tricbostrongylus colubriformis.Veterinary Record,
146:
728–732. TopBack | | BaeH. D., McAllisterT. A., YankeJ., ChengK. J., MuirA. D.
1993.
Effects of condensed tannins on endoglucanase activity and filter paper digestion by Fibrobacter succinogenes 585.
Applied and Environmental Microbiology,
59:
2132–2138. TopBack | | BarryT. N.
1989.
Condensed tannins: their role in ruminant protein and carbohydrate digestion and possible effects upon the rumen ecosystem. In:
The Roles of Protozoa and Fungi in Ruminant Digestion.
(Eds. NolanJ.V., LengR.A., DemeyerD.I.).
Penambul Books,
Armidale. pp
153–169. TopBack | | BarryT. N., DuncanS. J.
1984.
The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep. 1. Voluntary intake.
British Journal of Nutrition,
51:
485–491. TopBack | | BarryT. N., ManleyT. R.
1986.
Interrelationships between the concentration of total condensed tannins, free condensed tannins and lignin in Lotus spp. and their possible consequences in ruminant nutrition.
Journal of the Science of Food and Agriculture,
37:
248–254. TopBack | | BarryT. N., McNabbW. C.
1999.
The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants.
British Journal of Nutrition,
81:
263–272. TopBack | | BeeverD. E., SiddonsR. C.
1986.
Digestion and metabolism in the grazing ruminant. In:
Control of Digestion and Metabolism in Ruminants,
(Eds. MilliganP. P., GrovumW. L., DobsonA.).
Englewood Cliffs,
NJ, Prentice Hall. pp
479–497. TopBack | | BhatT. K., SinghB., SharmaO. P.
1998.
Microbial degradation of tannins - a current perspective.
Biodegradation,
9:
343–357. TopBack | | BhattaR., KrishnamoorthyU., MohammedF.
2000.
Effect of feeding tamarind (Tamarindus indica) seed husk as a source of tannin on dry matter intake, digestibility of nutrients and production performance of cross bred dairy cows in mid-lactation.
Animal Feed Science and Technology,
83:
67–74. TopBack | | BrownM. D., PoppiD. P., SykesA. R.
1991.
The effect of post ruminant infusion of protein or energy on the pathophysiology of Trichostrongylus colubriformis infection and body composition in lambs.
Australian Journal of Agricultural Research,
42:
253–267. TopBack | | ButlerL. G., RoglerJ. C.
1992.
Biochemical mechanism of the anti nutritional effects of tannin. In:
Phenolic Compounds in Food and their Effects on Health. 1. Analysis, Occurrence and Chemistry.
American Chemical Society,
Washington. pp
298–304. TopBack | | ButterN. L., DawsonI. M., WakelinD., ButteryP. J.
1998.
Effect of dietary tannins and protein level on the susceptibility of sheep to parasitic infections. In:
Proceedings of the British Society of Animal Science. p.
97. TopBack | | CoopR. L., HolmesP. H.
1996.
Nutrition and parasitic interaction.
International Journal for Parasitology,
26:
951–962. TopBack | | CoopR. L., KyriazakisI.
1999.
Nutrition parasitic interaction.
Veterinary Parasitology,
16:
1–18. TopBack | | FerreiraD., BrandtE. V., CoetzeeJ., MalanE.
1999.
Condensed tannins.
Progress in the Chemistry of Organic Natural Products,
77:
22–59. TopBack | | FillipichL. J., ZhuJ., OelrichsP., AlsalamiM.T., DoigA.J., CaoG.R., EnglishP.B.
1991.
Hepatotoxio and nephrotoxic principles in Terminalia oblongata.
Research in Veterinary Science,
50:
170–177. TopBack | | FooL. Y., NewmanR., WaghornG.C., McNabbW.C., UlyattM.J.
1996.
The proanthocyanidins from Lotus corniculatus.
Phytochemistry,
41617–624. TopBack | | FooL. Y., LuY., McNabbW.C., WaghornG.C., UlyattM.J.
1997.
Proanthocyanidin from Lotus pedunculatus.
Phytochemistry,
45:
1689–1696. TopBack | | GargS. K., MakkarH.P.S., NagalK.B., SharmaS.K., WadhwaD.R., SinghB.
1992.
Toxicological investigations into oak (Quercus incana) leaf poisoning in cattle.
Veterinary and Human Toxicology,
34:
161–164. TopBack | | HoskinS. O., WilsonP. R., BarryT. N., CharlestonW.A.G., WaghornG.C.
2000.
Effect of forage legume containing different condensed tannin content on internal parasites in young red deer (Cervus elaphus).
Research in Veterinary Science,
68:
223–230. TopBack | | JonesW. T., ManganJ. L.
1977.
Complexes of the condensed tannins of sainfoin with fraction 1 leaf protein and with submaxillary mucoprotein, and their reversal by polyethylene glycol and pH.
Journal of the Science of Food and Agriculture,
28:
126–136. TopBack | | JonesW. T., AnersonL. V., RossM. D.
1973;
Bloat in cattle. XXXIX. Detection of protein precipitant (flavolans) in legumes.
New Zealand Journal of Agricultural Research,
16:
441–446. TopBack | | JonesG. A., McAllisterT. A., MuirA. D., ChengK.J.
1994.
Effects of sainfoin (Onobrychis viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria.
Applied and Environmental Microbiology,
60:
1374–1378. TopBack | | KabasaJ. D., Opuda-AsiboJ., ter MeulenU.
2000.
The effect of oral administration of polyethylene glycol on faecal helminth egg counts in pregnant goats grazed on browse containing condensed tannins.
Tropical Animal Health and Production,
32:
73–86. TopBack | | KimamboA. E., MacRaeJ. C., WalkerA., WattC.F., CoopR.L.
1988.
The effect of prolonged subclinical infection with Trichostrongylus colubriformis on the performance and nitrogen metabolism of growing lambs.
Veterinary Parasitology,
28:
191–203. TopBack | | KumarR., SinghM.
1984.
Tannins: their adverse role in ruminant nutrition.
Journal of Agriculture and Food Chemistry,
32:
447–453. TopBack | | KumarR., VaithiyanathanS.
1990.
Occurrence, nutritional significance and effect on animal productivity of tannins in tree leaves.
Animal Feed Science and Technology,
30:
21–38. TopBack | | LiY. G., TannerG., LarkinT.
1996.
The DMCA-HCI protocol and the threshold proanthocyanidin content of bloat safety in forage legumes.
Journal of the Science of Food and Agriculture,
70:
89–101. TopBack | | LowryJ. B., McSweeneyC.S., PalmerB.
1996.
Changing perceptions of the effect of plant phenolics on nutrient supply in the ruminant.
Australian Journal of Agricultural Research,
47:
829–842. TopBack | | MacRaeJ. C.
1993.
Metabolic consequences of intestinal parasitism.
Proceedings of the Nutrition Society,
52:
121–130. TopBack | | MakkarH.P.S., SinghB., DawraR.K.
1987.
Tannin-nutrient interaction-a review.
International Journal of Animal Sciences,
2:
127–140. TopBack | | MakkarH.P.S., SinghB., DawraR.K.
1988.
Effect of tannin-rich leaves of oak (Quercus incana) on various microbial enzyme activities of the bovine rumen.
British Journal of Nutrition,
60:
287–29. TopBack | | ManganJ. L.
1959.
Bloat in cattle. XI. The foaming properties of protein, saponin and rumen liquor.
New Zealand Journal of Agricultural Research,
2:
47–61. TopBack | | ManganJ. L.
1988.
Nutritional effects of tannins in animal feeds.
Nutrition Research Reviews,
1:
209–231. TopBack | | MartinS. A., AkinD. E.
1988.
Effect of phenolic monomers on the growth and β - glucosidase activity of Bacteroides ruminicola and on the carboxymethylcellulase, β-glucosidase, and xylanase activities of Bacteroides succinogenes.
Applied and Environmental Microbiology,
54:
3600–3604. TopBack | | McLeodM. N.
1974.
Plant tannins-their role in forage quality.
Nutrition Abstracts and Reviews,
44:
803–815. TopBack | | McNabbW. C., WaghornG. C., PeterJ. S., BarryT. N.
1996.
The effect of condensed tannins in Lotus pedunculatus upon the solubilization and degradation of Ribulose 1–5 biphosphate carboxylase (EC 4.1.1.39; Rubisco) protein in the rumen and the sites of Rubisco digestion.
British Journal of Nutrition,
76:
535–549. TopBack | | McNabbW. C., PetersJ. S., FooL. Y., WaghornG. C., JacksonF. S.
1998.
Effect of condensed tannin prepared from several forages on the in vitro precipitation of Ribulose 1–5 biphosphate carboxylase (EC 4.1.1.39; Rubisco) protein and its digestion by trypsin (EC 2.4.21.4) and chymotrypsin (EC 2.4.21.1).
Journal of the Science of food and Agriculture,
77:
201–212. TopBack | | MinB. R., McNabbW. C., BarryT. N., KempP. D., WaghornG. C., McDonaldM. S.
1999.
The effect of condensed tannins in Lotus corniculatus upon reproductive efficiency and wool production in sheep during late summer and autumn.
Journal of Agricultural Science,
Cambridge.
(In press). TopBack | | MolanA. L., HoskinS. O., BarryT. N., McNabbW. C.
2000a.
Effect of condensed tannins extracted from four forages on the viability of the larvae of deer lungworm and gastrointestinal nematodes.
Veterinary Record,
147:
44–48. TopBack | | MolanA. L., DuncanA., BarryT. N., McNabbW. C.
2000b.
Effects of condensed tannins and sesquiterpene lactones extracted from chicory on the viability of the deer lungworm larvae.
Proceedings of the New Zealand Society of Animal Production,
60:
26–29. TopBack | | MolanA. L., WaghornG. C., MinB. R., McNabbW. C.
2000c.
The effects of condensed tannins from seven herbages on Trichostrongylus colubriformis larval migration in vitro.
Folia Parasitologica,
47:
39–44. TopBack | | MolanA. L., AlexanderR. A., BrookesI. M., McNabbW. C.
2000d.
Effects of an extract from sulla (Hedysarum coronarium) containing condensed tannins on the migration of three sheep gastrointestinal nematodes in vitro.
Proceedings of the New Zealand Society of Animal Production,
60:
21–25. TopBack | | NiezenJ. H., CharlestonW.A.G., HodgsonJ., WaghornT.S.
1993a.
Effect of four grass species on lamb parasitism and growth.
Proceedings of the New Zealand Grassland Association,
55:
203–206. TopBack | | NiezenJ. H., WaghornG. C., WaghornT. S., CharlestonW. A. G.
1993b.
Internal parasites and lamb production-role for plant containing condensed tannins.
Proceedings of the New Zealand Society of Animal Production,
53:
235–238. TopBack | | NiezenJ. H., WaghornT. S., RaufautkK., RobertsonH. A., McFarlaneR. G.
1994.
Lamb weight gain and faecal egg count when grazing one of seven herbages and dosed with larvae for six weeks.
Proceedings of the New Zealand Society of Animal Production,
54:
15–18. TopBack | | NiezenJ. H., WaghornT. S., CharlestonW. A. G., WaghornG. C.
1995.
Growth and gastrointestinal nematode parasitism in lambs grazing either lucerne (Medicago sativa) or Sulla (Hedysarum coronarium) which contains condensed tannins.
Journal of Agricultural Science, Cambridge,
125:
281–289. TopBack | | NiezenJ. H., CharlestonW.A.G., HodgsonJ., McKyA.D., LeathwickM.
1996.
Controlling internal parasites in grazing ruminants without recourse to anthelmintics: Approaches, experiences, prospects.
International Journal for Parasitology,
26:
983–992. TopBack | | NiezenJ. H., RobertsonH. A., WaghornG. C., CharlestonW. A. G.
1998.
Production, faecal egg count and worm burdens of ewe lambs which grazed six contrasting forages.
Veterinary Parasitology,
80:
15–27. TopBack | | ReedJ. D.
1995.
Nutritional toxicology of tannins and related polyphenols in forage legumes.
Journal of Animal Sciences,
73:
1516–1528. TopBack | | RobertsonH. A., NiezenJ. H., WaghornG. C., CharlestonW. A. G., JinlongM.
1995.
The effect of six herbages on liveweight gain, wool growth and faecal egg count of parasitised ewe lambs.
Proceedings of the New Zealand Society of Animal Production,
55:
199–201. TopBack | | TerrillT.H., DouglasG.B., FooteA.G., PurchasR.W., WilsonG.F., BarryT.N.
1992.
Effect of condensed tannins upon body growth, wool growth and rumen metabolism in sheep grazing sulla (Hedysarum coronarium) and perennial pasture.
Journal of Agricultural Science, Cambridge,
119:
265–273. TopBack | | WaghornG.C., UlyattM.J., JohnA., FisherM.T.
1987.
The effect of condensed tannins on the site of digestion of amino acids and other nutrients in sheep fed Lotus corniculatus.
British Journal of Nutrition,
57:
115–126. TopBack | | WaghornG. C., JonesW. T., SheltonI. D., McNabbW. C.
1990.
Condensed tannins and the nutritive value of herbage.
Proceedings of the New Zealand Grassl and Association,
51:
171–175. TopBack | | WaghornG. C., SheltonI. D., McNabbW. C., McCutcheonS. N.
1994.
Effects of condensed tannins in Lotus pedunculatus on its nutritive value for sheep. 2. Nitrogenous aspects.
Journal of Agricultural Science, Cambridge,
123:
109–119. TopBack | | WangY., DouglasG. B., WaghornG. C., BarryT. N., FooteA. G.
1996a.
Effect of condensed tannins in Lotus corniculatus upon lactation performance in ewes.
Journal of Agricultural Science, Cambridge,
126:
353–362. TopBack | | WangY., DouglasG. B., WaghornG. C., BarryT. N., FooteA. G., PurchasR. W.
1996b.
Effect of condensed tannins upon the performance of lambs grazing Lotus corniculatus and lucerne.
Journal of Agricultural Science, Cambridge,
126:
87–98. TopBack | | WoodwardA., ReedJ. D.
1997.
Nitrogen metabolism in sheep and goat consuming Acacia brevispica and Sesbania sesban.
Journal of Animal Science,
75:
1130–1139. TopBack | | WoodwardS. L., AuldistM. J., LaboyrieP. J., JansenE. B. L.
1999.
Effect of Lotus corniculatus and condensed tannins on milk yield and milk composition of dairy cows.
Proceedings of the New Zealand society of Animal Production,
59:
152–155. TopBack |
| |
|
|
|