Pathomorphological studies and detection of bovine papilloma viruses in wart-like lesions/growths in reticulum of buffaloes Jangir Babu Lal1,2, Bind R.B.2, Kumar Pawan2, Somvanshi R.2,* 2Division of Pathology, ICAR-Indian Veterinary Research Institute, Izatnagar-243122, Uttar Pradesh, India; 1Department of Veterinary Pathology, College of Veterinary Sciences, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar-125004, Haryana, India *Corresponding author: e-mail: dr.rsomvanshi@gmail.com
Abstract The present investigation was carried out to study the pathomorphology of wart-like lesions/growths in reticulum of buffaloes and their association with different bovine papilloma virus (BPV) types. A total of 19 tissue samples of reticulum were included in this study. Grossly, single to multiple, pin-head to pea-nut sized, sessile, pedunculated, dome shaped and mushroom-like growths with thickened reticular pillars were observed. Histopathologically, these were diagnosed as fibropapilloma, papilloma, hyperplasia and reticulitis. Ultrastructurally, the pleomorphic spinocytes containing variable shaped nuclei with scanty heterochromatin and distinct nuclear membrane were observed. PCR revealed presence of BPV-1, -2 and -5 DNA alone or their mixed infections. Quantitative real-time PCR revealed varying amount of viral DNA copy numbers of BPV-1, -2 and -5. Immunostaining for Ki-67 in fibropapilloma cases varied with random distribution of the immunopositive cells. As compared to basal cells; more number of Ki-67 immunopositive cells was observed in suprabasal cells. Cyclooxygenase-2 (COX-2) expression showed cytoplasmic staining with occasional membrane staining, especially in koilocytes. The stromal inflammatory cells in reticulitis associated cases were also moderately positive for COX-2 immunostaining. In conclusion, BPV-1, -2 and -5 were associated either alone or their mixed infections with reticular wart-like lesions/growths in buffaloes. The basal and suprabasal cells of the reticular fibropapilloma were cellular proliferation sites. In conclusion, the present investigation revealed that the growths/wart-like lesions occurring in the reticulum of slaughtered buffaloes are associated with infection of BPV-1, -2 and -5, as DNA of these viruses were detected in such cases. Top Keywords Bovine papilloma virus, Buffalo, Ki-67, Reticulum, Warts. Top |
INTRODUCTION Traumatic reticulo-pericarditis or hard ware disease is known to be well studied pathological condition of reticulum. However, other pathological conditions of reticulum are not sytemically studied. Few cases of spontaneous tumours of reticulum were described from India. Sporadic cases of squamous cell carcinoma were reported in rumen and reticulum of Haryana bullocks1,2 and papilloma in rumen of buffaloes3. A classical case of warts with multiple growths in reticulum of a buffalo was also reported from our laboratory in which the DNA of bovine papillomavirus (BPV) was detected by polymerase chain reaction (PCR)4. This finding leads to design a special study for undertaking systematic investigation on reticular wart-like lesions in Indian buffaloes. |
BPV is a member of double stranded DNA virus family, papillomaviridae. The BPV infection is clinically characterized by typical hyperproliferative lesions or papilloma on cutaneous tissues and mucosa5. At present, 14 different BPV types have been characterized and found to be associated with papillomatosis in different body regions6. The BPV-4 is mainly associated with papillomas of the mouth, oesophagus and rumen7,8-9. However; BPV -2 had been also reported from the fibropapillomas of oesophagus and rumen10. In India, the association of BPV-1 and -2 with warts in cattle, buffaloes, yak; skin lesions, sarcoids and tumours in equines were studied extensively11,12,13-14. The co-infection of different BPV types has been reported in wart-like lesions in cattle and buffaloes from India and various parts of world11,12,15,16. |
The cellular proliferation markers were found to be expressed in papillomas/fibropapillomas3,12,17. Ki-67 is expressed after cell stimulation in the G1-phase. It remains immunohistologically detectable throughout the interphase of cell division and reaching its maximal level of expression during mitosis18. Another marker, cyclooxygenase-2 (COX-2) is a key enzyme involved in the modulation of inflammatory process, it catalyses the rate limiting step in the formation of prostaglandins from arachidonic acid and was up regulated in many pathological conditions including carcinogenesis19. COX-2 over expression is reported in several epithelial neoplasms, including in cattle, canines and human urothelial neoplasms and levels of its expression have been shown correlation with invasiveness and prognosis in some tumours which suggests important role of COX-2 in tumour development and progression20,21-22. |
Recent reports indicated that BPV infection is not site- specific and mixed infections of different BPV types have been detected in cutaneous and mucosal warts of cattle and buffaloes from different parts of the world5. Keeping this in view, the present investigation was carried out to study the pathomorphology and association of different BPV types in wart-like lesions/growths in reticulum of buffaloes. |
Top MATERIALS AND METHODS Samples The suspected tissue samples of wart-like lesions/growths in reticulum (17 growths/wart-like lesions; 2 normal reticulum) were collected from different buffalo slaughter houses located in North Indian cities namely, Bareilly (Uttar Pradesh) and Haldwani (Uttarakhand). These samples were preserved in 10% buffered formalin for histopathological and immunohistochemical studies, 2.5% glutraldehyde for transmission electron microscopy (TEM) and -20°C for molecular studies. Pathomorphological studies The detailed gross observations with specific locations of growths/wart-like lesions were recorded. After proper fixation, the tissues were processed for histopathology by standard procedure. Briefly, tissues were dehydrated in ascending grades of alcohol, cleared in xylene followed by paraffin embedding for preparation of blocks. The sections with 4–5μm thickness were cut and subjected to haematoxylin and eosin (H&E) staining for histopathological study as per the conventional procedure. Ultrastructural studies For TEM studies, fresh tissue samples were collected and preserved as per standard procedure. Briefly; pieces of 1 mm2 were preserved in 2.5% gluteraldehyde in 0.2 M phosphate buffer (pH 7.4) at 4°C. The tissue processing for TEM studies were carried out using standard procedure. The tissue pieces were washed with pre chilled 0.2 M phosphate buffer (pH 7.4; three changes for 2 hours each), fixed with 1% Osmium tetroxide, dehydrated in ethyl alcohol, cleared and embedded in epon araldite resin. Ultra thin sections were cut by ultra microtome (Ultracut Reichert-Jung, Austria) and mounted on copper grids and stained with uranyl acetate and lead citrate. The tissue grids were examined under electron microscope (TECNAI G20, FEI Company, Netherland). From tissue processing to TEM, work was carried out at Electron Microscope Facility, Department of Anatomy, All India Institute of Medical Sciences, New Delhi by standard protocol. Molecular studies DNA extraction and primers DNA was extracted with Genomic DNA Mini Kit (Qiagen, Hilden, Germany) as per the manufacturer‘s instructions from frozen tissue samples stored at −20°C. The primers pairs, BPV-1 (forward 5'-ggagcgcctgctaactatagga-3', reverse 5'-atctgttgtttgggtggt- gac-3'); BPV-2 (forward 5'-gttataccacccaaagaagaccct-3', reverse 5'-ctggttgcaacagctctctttctc-3'); BPV-5 (forward 5'-actggctctaccaagctcaagg-3', reverse 5'-gacagaagggtta- acggtctgca-3') and BPV-10 (forward 5'-tgcattcaatagg- cttgcagatgc-3', reverse 5'-cacctcgagaccacaaatgc-3') were used. These primer pairs were expected to amplify the specific viral DNA template of L1 genes of sizes 301, 164, 266 and 422bp, respectively. The primer pair of BPV-5 (forward 5'-ggcagtacacaagctaggcca-3', reverse 5'-tgcaggattcacatccaaaacagc-3') was expected to amplify the specific viral DNA template of L2 gene of size 360 bp. Specific primers for BPV-1 and -2 were used as per previous workers23. However, the specific primers for BPV-5 and -10 were designed by using published sequences with the help of primer designing software (DNASTAR software, DNASTAR, Madison, WI, USA). PCR and sequencing For PCR, the initial denaturation was carried out at 94°C for 3 minutes (min). This was followed by 35 cycles of denaturation at 94°C for 40 seconds (sec), annealing at 52°C (BPV-1 and -2), 62°C (BPV-5) and 60°C (BPV-10) for 40 sec, with a 50 sec extension at 72°C and single cycle of final elongation of 10 min at 72°C. The amplified DNA fragments were visualized by transillumination under UV light (Geldoc; Alpha Tech, San Leandro, CA, USA) in 1.5% agarose containing ethidium bromide (0.5 g/ml). The representative PCR products were sequenced commercially at Department of Biochemistry, Delhi University, South Campus, New Delhi on ABI-PRISM dye terminator DNA Sequencing Facility. The sequencing data was analysed by EDITSEQ and MegAlign modules of LASERGENE software (DNASTAR). The confirmation of generated sequences in present study was done by aligning them by the clustal method with the published sequences of different BPVs. Quantitative real-time PCR assay For quantitative realtime PCR assay, the same primer pairs were used as for conventional PCR assay. It was performed in Stratagene Mx-3000 real-time PCR cycler by using commercial reagents as per the manufacturer‘s recommendations. The reaction set up was comprised of 25μl of Eva Green qPCR master mix, 0.6μl each of forward and reverse primers, 3.0μl DNA and nuclease free water to make a total reaction volume of 50μl. The thermal profile for BPV-1 comprised of three steps. In step 1, initial denaturation was carried out at 95°C for 10 min followed by step 2 comprised of 40 repetitive cycles of denaturation at 94°C for 10 sec, annealing at 55°C for 25 sec and extension at 72°C for 30 sec each. The dissociation curve analysis was performed in the step 3 which comprised of a single cycle of denaturation at 95°C for 1 min, partial renaturation to 65°C for 30 sec and denaturation again at 95°C for 30 sec. For BPV-2 and -5, the thermal profile was comprised of similar three steps with annealing at 60°C and 63°C for 30 and 20 sec, respectively. For BPV- 1,-2 and -5, the purified PCR products of cattle cutaneous warts were used as standards. Using nanodrop, the DNA concentration was measured in these samples. Then, by using available on line softwares copy numbers were calculated. Standard curves were obtained by 10-fold serial dilutions of purified PCR products. Immunohistochemistry (IHC) For IHC, thetissue sections were taken on 3-aminopropyl-triethoxysilane coated slides (Sigma Chemicals, Sigma, St. Louis, MO, USA). Sections were deparaffinized and rehydrated in xylene and descending grades of alcohol, respectively. Antigen retrieval was performed with microwave heating in 0.01 M citrate buffer pH 6.0, twice for 5 min each. Then tissue sections were incubated in 3% H2O2 in methanol for 30 min for blocking of endogenous peroxidase activity. After thoroughly rinsing with PBS (pH 7.2), the non-specific sites were blocked by incubating each section with 5% normal goat serum (Sigma Chemicals) and 1.5% donkey serum (Santa Cruz Biotech) in PBS for 30 min for Ki-67 and COX-2 expression, respectively. Immunostaining was performed with primary antibodies, mouse monoclonal anti-Ki67 (mAb PP-67, P6834, Sigma Chemicals, USA) and goat polyclonal anti-COX-2 IgG antibody (SC-1746; Santa Cruz Biotech) applied overnight at 4°C at 1:50 dilution in PBS containing 1% bovine serum albumin. The negative controls were covered with diluent only. Then sections were washed three times with PBS and incubated with the biotinylated secondary antibody and peroxidase according to the manufacturer‘s instructions (Sigma for Ki-67 and Santa Cruz Biotech for COX-2). In between the sections were washed three times with PBS. Then, moist sections were covered with sufficient 3-amino-9-ethyl-carbazole (AEC; Sigma Chemicals) staining substrate for 3–10 min and counterstained lightly with Mayer7 s haematoxylin (MHS- 16; Sigma). After rinsing in running tap water for 5 min, the sections were mounted in glycerol gelatin. Using light microscope, the sections were evaluated and staining intensity of the tumour cells was classified semi-quantitatively. The Ki-67 labelling index was determined by counting at least 1,000 tumour cells and the index was calculated as a percentage. First of all, the entire section was screened to find the region with the maximum number of positively stained nuclei in one microscopic field (magnification, 40× objectives). The fraction of immunopositive cells for Ki-67 was counted as described previously24. The COX-2 staining distribution score was defined by the estimated percentage of positive stained cells in five 40× fields as described earlier25. The percentage of positive tumour cells was determined semiquantitatively by assessing the whole tumour section. The COX-2 staining intensity was defined as the strength of the signal in positive stained tumours, with no signal (−, 0), weak signal (+, 1), moderate signal (++, 2) and strong signal (+++, 3). Top RESULTS Pathomorphological studies In present investigation, a total of 19 samples (17 wart-like lesions/growths in reticulum and 2 normal reticulum or healthy control) were studied. Grossly, single to multiple, pin-head to pea-nut sized, sessile, pedunculated, dome shaped and mushroom-like growths with thickened reticular pillars were observed (Fig. 1a, b). The detailed gross observations are presented in Table 1. On histopathological examination, these were diagnosed as fibropapilloma, papilloma, hyperplasia, reticulitis and normal reticulum (Table 1). The growths/wart-like lesions diagnosed as fibropapilloma were characterized by parakeratosis, hyperkeratosis and acanthosis with distinct vacuolating cells (koilocytes). It also revealed medium to large sized rete pegs with well developed fibrous connective tissue cores and were deeply inserted into proliferating fibrous connective tissue (Fig. 2a). Occasionally, mitotic figures were also present. In two cases, in addition to above changes encapsulated granuloma-like lesions with infiltration of mononuclear cells (MNCs) and neutrophils were observed. A few foreign body giant cells were also present. These cases were diagnosed as fibropapilloma with granulomatous reticulitis (Figs. 2b, c). Microscopically, papillomas were characterized by well developed irregular shaped retepegs with hyperkeratosis, parakeratosis and negligible to mild fibrocellular reaction (Fig. 2d). Epithelial hyperplasia was characterized by presence of mild acanthosis, a few koilocytes and transformed basal cells with indistinct retepeg formation. Another two cases revealed similar histopathological findings with severe MNCs infiltration and occasionally, neutrophils. It also revealed angiogenesis with newly formed blood capillaries. These cases were diagnosed as reticulitis. Tissue samples from normal reticulum revealed the normal architecture with presence of different layers. Ultrastructural study Five cases of reticular warts (three cases of fibropapilloma, one case each of hyperplasia and normal reticulum) were studied ultrastructurally. Ultrastructurally, fibropapilloma cases revealed pleomorphic spinocytes with variable shaped nuclei (round/oval/elongated/fish/D shaped) containing scanty heterochromatin and distinct nuclear membrane. However, some of the nuclei revealed indistinct nuclear membrane. Marked intercellular spaces and microvilli connecting intercellular membranes were also observed. Around these cells large vacuoles, cytoplasmic debris or cytoplasm with vacuoles were seen. Nuclei were enlarged and contained prominent nucleoli. In some of the nuclei, nucleoli were marginally placed. The cytoplasmic organelles were not observed (Fig. 3a, b, c). On ultrastructure study, the histopathologically diagnosed case of hyperplasia, revealed electron dense pleomorphic spinocytes with prominent intercellular spaces and interconnecting thick bands/microvilli on cytoplasmic membranes. Nuclei were oval or elongated shaped and contained centrally placed electron-dense prominent nucleolus. Heterochromatin was moderate with an intracytoplasmic inclusion with a clear halo. Cytoplasm was scanty and electron dense in nature and it contained few indistinct mitochondria and numerous RER. Other cytoplasmic organelles were not distinct (Fig. 3d). BPV virus-like particles were not observed in any of the cases. Ultrastructural study of the histologically normal reticulum showed epithelial cells with microvilli on their cytoplasmic membranes connecting with adjacent cells. Nuclei were oval to elongate in shape with distinct nuclear membrane. Cytoplasm was indistinct with a few indistinct cell organelles. In some cells distinct ER was seen. PCR and sequencing A total of 19 samples were subjected to PCR. Out of 19 samples, 4 samples were positive for BPV-1, 2 for BPV-2 and 3 for BPV-5 alone while 2 samples were positive for the mixed infection of BPV-1 & -2 and 1 for BPV-1 & -5. A total of 7 samples were negative for DNA of BPV-1, -2 & -5 alone or their mixed infections. None of the samples were positive for DNA of BPV-10. Gel electrophoresis showed the specific PCR product sizes of 301, 164 and 266 bp for BPV-1, -2 and -5, respectively (Fig. 4a, b). Selective PCR products were sequenced and the generated sequences were submitted to NCBI and EMBL data-base and accession numbers were assigned (KF148689, HG007971, HG918264 and HG794238). Quantitative real-time PCR assay DNA samples positive for different BPV types by PCR were utilized for determination of viral DNA copy numbers present in them by using quantitative real-time PCR. These samples were tested together with known standard samples along with “No Template Control” (NTC). The copy numbers of BPV-1, BPV-2 and BPV-5 in reticular warts varied from 1.257e+003 to 2.148e+005, 6.398e+006 to 1.815e+008 and 3.539e+003 to 3.301e+005, respectively. Immunohistochemistry Sixteen samples of reticular wart-like lesions were studied by IHC for expression of Ki-67 and COX-2. Scoring of Ki-67 and COX-2 in different cases is presented in Table 2. A total of 5/16 (31.25%) samples showed positive immunostaining for Ki-67. Immunopositivity for Ki-67 was noticed in only three (3) cases of fibropapilloma, one (1) case each of hyperplasia and fibropapilloma with reticulitis. An immunopositive Ki-67 nuclear staining with moderate intensity was observed in basal cells of hyperplastic epithelial cells. However, only a few fibroblastic cells showed positive reactivity for Ki-67. In reticulitis case, few inflammatory cells as well as proliferating fibroblasts showed nuclear staining for Ki-67. In fibropapilloma cases, immunostaining for Ki-67 were varied with random distribution of the immunopositive cells. As compared to cells of basal layer, more number of the Ki-67 immunopositive cells was observed in suprabasal cells, particularly, in the spinosum cells. Cells at the periphery of fibrous connective tissue cores showed intense nuclear staining as compared to spinosum cells (Fig. 5a, b). Positive immunoreactivity for COX-2 was observed in 3/16 (18.75%) cases. COX-2 immunostaining was observed in fibropapilloma and reticulitis cases. Its expression showed cytoplasmic staining, however, occasionally membrane staining was observed especially in koilocytes. The stromal inflammatory cells, in cases with associated reticulitis, were also moderately positive for COX-2 immunostaining (Fig. 5c, d). Top DISCUSSION Pathological conditions of reticulum are not well studied except reticulitis in relation with traumatic reticulo pericarditis. Few cases of spontaneous tumours of reticulum are described from India including a case of reticular warts with multiple growths in reticulum of a buffalo where BPV was detected4. This case created our interest for undertaking detailed systematic investigation on reticular wart-like lesions/growths in slaughtered Indian buffaloes. The small growths in reticulum seemed to be irrelevant to produce any functional defects, however, if growths are extensive; it may lead to discomfort and gastro-intestinal functional disorders. The warts usually developed at places where stratified squamous epithelium is present; therefore, fore-stomach having such type of epithelium is susceptible to induction of warts. In an extensive survey of gastrointestinal papillomas of cattle in the North-Western Highlands of Scotland florid type of papillomas, widespread in distribution, persistent and often accompanied by squamous cell carcinomas, at the same anatomical sites were reported26. Feeding of bracken fern to animals affected with gastrointestinal warts may convert papillomas into to carcinomas27. The reticular warts were not studied earlier in buffaloes from non-enzootic areas. |
Histopathologically, the wart-like lesions/growths of reticulum were diagnosed as papilloma, fibropapilloma, hyperplasia and reticulitis. This is in accordance with the findings of earlier workers3,4. Reticulum is predilection site of sharp edge hardware objects which may cause chronic irritation and traumatic injury leading to reticulitis. This may further progress to induction of warts or neoplastic conditions. In present investigation occasionally conditions like- reticulitis/granulomatous reticulitis were also observed, which suggest that role of trauma/injury in such type of lesions must be ruled out. |
Till date in literature none of the study has described the ultrastructure of warts of reticulum origin. However, the ultrastructural changes observed in reticular warts in present study were similar to that observed in ruminal wart-like lesions (unpublished data) as well as with those described for papillomatosis in cattle and buffaloes by earlier workers28,29-30. None of the samples examined ultrastructurally revealed BPV-like particles. This is similar to observations made in mucosal tumours of urinary bladders in enzootic bovine haematuria cases where BPV-1/-2 infects the epithelium of the bladder and produces an abortive or latent infection31. |
This is the first report of reticular wart-like lesions in buffaloes showing the presence of BPV-5 DNA by PCR; however, BPV-1 and -2 DNA have been detected in a case of reticular warts by earlier workers from our laboratory4. Papillomas of the upper gastrointestinal tract such as mouth, oesophagus and rumen are associated with BPV-47,8. However, BPV-4 and- 5 were detected in the squamous papillomas obtained from bovine facial skin; although it was believed that BPV-4 and -5 infect gastrointestinal tract and teat/udder skin, respectively32,33. The mixed infections of 2–4 different types of BPVs were reported in Brazilian cattle and it was concluded that different BPV types were not site-specific and mixed infection may be responsible for persistence of lesions. BPV-1, -2 and -5 DNA was detected in the wart-like lesions of gastrointestinal tract which is an uncommon site for these viruses3. From these observations, it may be inferred that more types of BPVs/mixed infection occurs in cattle and buffaloes similar to that of human beings by human papilloma viruses (HPVs). More investigations are desired in wider geographical areas with aim to detect different BPV types involved in such lesions. |
Quantitative real-time PCR revealed varying amount of viral DNA copy numbers of BPV-1, -2 and -5 in the PCR positive samples. In present study, on comparing the detected virus load among themselves, the copy numbers of viral DNA ofBPV-1 were found to be low, while that of BPV-5 and -2 varied from moderate to high, respectively. However, the earlier studies on cutaneous warts of cattle and buffaloes revealed high copy numbers of BPV-1 and-2 as compared to mucosal warts12. |
Immunostaining for Ki-67 in fibropapilloma cases were varied with random distribution of the immunopositive cells. As compared to cells of basal layer, more number of the Ki-67 immunopositive cells was observed in suprabasal cells. The pattern of the immunostaining for Ki-67 was almost similar to that of observed in cutaneous and mucosal warts of rumen which indicates similar basic pathogenesis mechanism of lesion3,12. In present study, the pattern of COX-2 immunostaining in mucosal warts of reticulum was similar to the HPV-positive cervical squamous intraepithelial lesions34. The immunopositivity in stromal cells was similar to that reported by earlier workers25. On the basis of the present findings and the available reports in literature, the over expression of COX-2 in high-grade lesions and low or no expression in benign lesions suggests that it plays a role in tumour progression rather than tumour initiation35. |
In conclusion, the present investigation revealed that the growths/wart-like lesions occur in the reticulum of slaughtered buffaloes are associated with infection of BPV-1, -2 and -5; as DNA of these viruses were detected in such cases. The cells of basal and suprabasal layers of the reticular fibropapilloma were cellular proliferation sites as they had higher expression of Ki-67. The BPVs may be entering in reticular epithelium through sharp objects found in reticulum and also producing traumatic reticulitis. |
Top ACKNOWLEDGEMENTS The authors are thankful to the Director, ICAR-Indian Veterinary Research Institute, Izatnagar, UP for providing necessary facilities. The first author is grateful to the Indian Council of Agricultural Research, New Delhi, for providing scholarship during the course of study. |
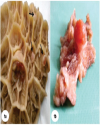
Top Figures | Fig. 1.: (a). Multiple mushroom-like growths (black arrows) attached to the mucosal surface of reticulum; (b). A large dome shaped wart-like growth with short stalk attached to mucosal surface of reticulum
|  | |
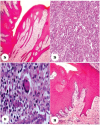
| | Fig. 2.: (a). Fibropapilloma showing elongated variable length retepegs deeply inserted in proliferating fibrous connective tissue. H&E ×100;(b). Reticulitis characterized by marked mononuclear cells infiltration in proliferating fibrous connective tissue. H&E ×100;(c). Granulomatous reticulitis showing infiltration of mononuclear cells and a giant cell. H&E ×400; (d). Papilloma characterized by acanthosis and well developed irregular shaped retepegs. H&Ex100.
|  | |
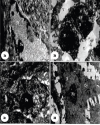
| | Fig. 3.: (a). Part of elongated electron-light nucleus (N) with marginally placed nucleolus (Nu). It contained electron dense cytoplasm (Cy), indistinct RER and tonofilaments (Tf). Fibropapilloma, TEMx4000; (b). Two D shaped cells containing large apoptotic bizarre shaped nuclei (N) with dispersed heterochromatin throughout the nuclei. Cytoplasm is electron-light and scanty containing several vacuoles with indistinct cytoplasmic membrane. These cells are surrounded by large vacuoles (V). Fibropapilloma, TEMx2000; (c). Part of cell showing fish shaped nuclei with enlarged, electron dense, marginally placed nucleolus (Nu). Space is seen between nucleus and cytoplasm. Cytoplasm (Cy) is scanty with absence of cell organelles. Fibropapilloma, TEMx4000; (d). A stellate shaped semi-electron dense spinocyte with enlarged elongated nucleus (N) containing centrally placed electron dense prominent nucleolus (Nu). On cytoplasmic membrane interconnecting thick bands/microvilli (Mv) and prominent intercellular spaces (ICS) are seen. Hyperplasia, TEMx3200
|  | |
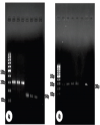
| | Fig. 4.: (a). PCR products in ethidium bromide stained 1.5% agarose TBE gel electrophoresis. M-100 bp marker; L1 (positive control), L2-L3 (re-ticular warts) BPV-1, 301 bp; L4 (positive control), L5-L6 (reticular warts) BPV-2, 164 bp and L7 (no template control); (b). PCR products in ethidium bromide stained 1.5% agarose TBE gel electrophoresis. M-100 bp marker; L1-L4 (reticular warts) & L6 (positive control), BPV-5, 266 bp and L7 (no template control).
|  | |
| | Fig. 5.: (a). Diffused intense intranuclear Ki-67 immunostaining in basal and suprabasal layer. Fibropapilloma with reticulitis, Ki-67, IHC, IPO-AEC-Mayer‘s Haematoxylinx100; (b). Higher magnification of Fig.5a exhibiting Ki-67 immunopositivity in suprabasal layer. Fibro-papilloma with reticulitis, Ki-67, IHC, IPO-AEC-Mayer‘s Haematoxylinx400; (c). Moderately intracytoplasmic COX-2 immunostaining in inflammatory cells. Reticulitis, COX-2, IHC, IPO-AEC-Mayer‘s Haematoxylinx100; (d). Diffused intracytoplasmic COX-2 immunopositive staining in inflammatory cells (inset) and fibroblasts. Fibropapilloma with reticulitis, COX-2, IHC, IPO-AEC-Mayer‘s Haematoxylinx400.
|  | |
|
Tables | Table 1.: Gross observations, histopathological diagnosis and detection of BPV types in wart-like lesions/growths in reticulum of buffaloes
| | S.No. | Samples | Gross Observations | Histopathological Diagnosis | PCR Results (BPV-1, BPV-2 BPV-5 & BPV-10 | | 1. | RET-1 | Eight small mushroom shaped growths in base of honey comb lattice | Fibropapilloma | + + - | | 2. | RET-2 | Three small wart-like growths on pillars | Fibropapilloma | + | | | 3. | RET-3 | Numerous small pin-head sized raised growths on pillars of honey comb lattice | Fibropapilloma | + - - - | | 4. | RET-4 | 2–3 elevated growths on reticular pillars | Hyperplasia+Reticulitis | - - - - | | 5. | RET-5 | Three growths of 2–4 mm diameter in size and 5–6 pin-head sized growths on pillars of honey comb lattice | Fibropapilloma+Reticulitis | - - - - | | 6. | RET-6 | One big wart-like growth of 5 mm in diameter at base of honey comb lattice and four small growths of approx. 2–3 mm in diameter | Hyperplasia + Reticulitis | + - - - | | 7. | RET-7 | One pedunculated growth of approx. 5 mm in diameter | Fibropapilloma | - - - - | | 8. | RET-8 | Three pin-head sized growths at the base of honey comb lattice | Fibropapilloma | - - + - | | 9. | RET-9 | 7–8 variable sized (approx. 2–3 mm diameter) wart-like growths at the base of honey comb lattice | Fibropapilloma | - - + - | | 10. | RET-10 | 3–4 small sized round wart-like lesions at borders of pillars+ Granulomatous reticulitis | Papilloma | + - - - | | 11. | RET-11 | One big and 6–8 small variable sized wart-like growths on the base of honey comb lattice | Fibropapilloma | + - +- | | 12. | RET-12 | Five variable sized (2–4 mm in diameter) growths on the base of honey comb lattice | Fibropapilloma | - + - - | | 13. | RET-13 | Four small variable sized (1–2 mm in diameter) wart-like lesions on the base of honey comb lattice | Papilloma | - - - - | | 14. | RET-14 | Two pin head-sized wart-like lesions on the base of honey comb lattice | Fibropapilloma | - + - - | | 15. | RET-15 | Three variable sized (2–4 mm in diameter) wart-like lesions on the base of honey comb lattice | Fibropapilloma | - - + - | | 16. | RET-16 | Single growth on the base of honey comb lattice | TNP | - - - - | | 17. | RET-17 | Two growths, one large pedunculated on the base of honey comb lattice and another one was small pin-head sized wart on the base of honey comb lattice | Fibropapilloma + Reticulitis | + + - - | | 18. | RET-18 | Normal reticulum | Normal | - - - - | | 19. | RET-19 | Normal reticulum | Normal | - - - - |
|
| TNP: Tissue Not Processed | | | Table 2.: Scoring of Ki-67 and COX-2 expression in wart-like lesions of reticulum
| | S. No. | Sample | Histopathological Diagnosis | Ki-67 Expression | COX-2 Expression | | Ki-max (%) | Ki-tot (%) | Positive Cells | Intensity (%) | | 1. | RET-1 | Fibropapilloma | 19.5 | 16.87 | - | - | | 2. | RET-2 | Fibropapilloma | - | - | - | - | | 3. | RET-3 | Fibropapilloma | - | - | 16.80 | ++ (2) | | 4. | RET-6 | Hyperplasia+ Reticulitis | 24.30 | 16.40 | 12.50 | ++ (2) | | 5. | RET-7 | Fibropapilloma | - | - | - | - | | 6. | RET-8 | Fibropapilloma | 17.33 | 14.87 | - | - | | 7. | RET-9 | Fibropapilloma | 9.60 | 7.40 | - | - | | 8. | RET-10 | Papilloma + Granulomatous reticulitis | − | − | − | − | | 9. | RET-11 | Fibropapilloma | - | - | - | - | | 10. | RET-12 | Fibropapilloma | - | - | - | - | | 11. | RET-13 | Papilloma | - | - | - | - | | 12. | RET-14 | Fibropapilloma | - | - | - | - | | 13. | RET-15 | Fibropapilloma | - | - | - | - | | 14. | RET-17 | Fibropapilloma+ Reticulitis | 13.66 | 9.40 | 23.20 | ++ (2) | | 15. | RET-18 | Normal | - | - | - | - | | 16. | RET-19 | Normal | - | - | - | - |
| |
|