Protective effects of neem (Azadirachta indica) leaf powder on experimentally induced colibacillosis in broilers Patil V.B.4, Khan M.A.4,*, Chavhan S.G.4, Gaikwad N.Z.1,4, Bhonsle A.V.2,4, Patil M.K.3,4 4Department of Pathology, College of Veterinary and Animal Sciences, Udgir, Maharashtra-413517, India 1Biochemistry, College of Veterinary and Animal Sciences, Udgir, Maharashtra-413517, India 2Microbiology, College of Veterinary and Animal Sciences, Udgir, Maharashtra-413517, India 3Pharmacology, College of Veterinary and Animal Sciences, Udgir, Maharashtra-413517, India *Corresponding author: e-mail: drminhajali@rediffmail.com
Abstract The present study was conducted to evaluate the preventive effect of neem (Azadirachta indica) leaf powder (NLP) in experimentally induced colibacillosis in broilers. Birds from T1 group {E. coli @ 0.3 ml (1 × 109 CFU/ml), single dose orally at the age of 7th day} showed clinical signs mainly such as respiratory distress, whitish pasty watery diarrhoea and high mortality (24%). The T1 group birds showed gross lesions mainly consisting marked thick yellowish fibrinous deposits on pericardium or epicardium and hepatic capsule. Histo- pathological lesions observed in T1 group were fibrino-heterophilic hepatic serositis and epicarditis or pericarditis. The capsular surface of affected liver and epicardial or pericardial surface of affected hearts were covered with thick layer of fibrin containing predominantly heterophils and numerous bacterial colonies. The marked hepatocellular degeneration and disruption of cardiomyocytes was also evident. The birds from T2 group (E. coli + Neem Leaf Powder @ 2 gm per kg of feed) showed comparatively lesser severity of clinical signs, gross or histopathological lesions and mortality (12%) than T1 group. In conclusion, the oral supplementation of neem leaf powder @2 gm per kg of feed although could not completely prevent the birds from E. coli infection, it induced moderate improvement in colibacillosis affected birds in terms of comparatively lesser severity of clinical signs, gross or histopathological lesions and reduction in mortality. Top Keywords Broilers, Colibacillosis, Neem leaf powder. Top |
INTRODUCTION Bacterial diseases of poultry represent a major threat to poultry industry all over the world and microbial infections are the world‘s leading killing diseases among the poultry. Colibacillosis is a broad term that refers to any infection or disease caused by the bacteria E. coli which continues to be one of the most economically important respiratory and systemic diseases leading to high morbidity, mortality, loss of body weight, poor feed conversion ratio (FCR), decrease in egg and meat production and condemning or low grading of carcasses. Colibacillosis is associated with many different kinds of diseases ranging from respiratory tract infection, swollen head syndrome in poultry to urinary tract infections1. Colibacillosis refers to any localized or systemic infection caused entirely or partly by avian pathogenic Escherichia coli (APEC), including colisepticemia, coligranuloma (Hjarre‘s disease), air sac disease (chronic respiratory disease, CRD), swollen-head syndrome, venereal colibacillosis, and coliform cellulitis (inflammatory process), peritonitis, salpingitis, orchitis, osteomyelitis/synovitis (turkey osteomyelitis complex), panophthalmitis, omphalitis/yolk sac infection, and enteritis2. |
Many synthetic drugs and growth promoters are supplemented to the broilers to effect rapid growth but their use have shown many disadvantages like high cost, adverse side effects on health of birds and long residual properties3. Exploring new antibiotics from the medicinal plants is a priority in this era of antibiotic resistance to increase the production of broilers. Various plant extracts have been used worldwide for a range of medicinal properties-like antibacterial, antiviral, antifungal, antiprotozoal and hepato-protective without adverse effects4,5. Therefore, the antibiotic selection pressure for resistance in bacteria in poultry is high and consequently their faecal flora contains a relatively high proportion of resistant bacteria6. One of such plants, Neem (Azadirachta indica) is an indigenous plant of Asian subcontinent known for its useful medicinal properties since ancient times. Neem has vast range of medicinal propertieslike antibacterial, antiviral, antifungal, antiprotozoal, hepatoprotective, anticancer and various other properties without showing any adverse effects. A. indica is a fast growing evergreen tree which has a potential to provide medicinal and nutritive value to broilers7. Hence, the present study was designed to study the protective effect of neem (A. indica) leaf powder on experimental induced colibacillosis in broilers. |
Top MATERIALS AND METHODS Experimental design An experiment was conducted on 100 day old broiler chicks. The experimental birds were randomly divided equally (n=25) into four groups (C1, C2, T1 and T2). Group C1 chicks were fed on normal poultry feed which served as healthy control. Group C2 chicks were fed on normal poultry feed mixed with neem (Azadirachta indica) leaf powder @ 2 gm/kg of feed for 35 days, which served as neem leaf powder (NLP) control. Group T1 birds were orally administered with nutrient broth culture of E. coli @ 0.3 ml (1 × 109CFU/ml) at the age of 7th day. The group T2 chicks were fed on normal poultry feed mixed with neem (A. indica) leaf powder @ 2 gm/kg of feed and on 7th day age, these birds were orally administered with nutrient broth culture of E. coli @ 0.3 ml (1×109CFU/ml). The experimental trial was approved by the Institutional Animal Ethics Committee (IAEC) and trial was conducted as per their guidelines. Preparation of Neem leaf powder Fresh leaves of the neem tree were collected from College Campus and shade dried for 4–6 days. The leaves were turned regularly to prevent uneven drying and decay. The shade dried leaves were powdered by using mixer and stored in airtight bags until use. Maintenance of E. coli culture and preparation of dose Pure culture of known (typed) pathogenic strain of E.coli was obtained from the Microbial Type Culture Collection and Gene Bank-Institute of Microbial Technology, Chandigarh (India) and maintained on MacConkey‘s Agar and Eosin Methylene Blue Agar. The standard E. coli culture were sub cultured using nutrient broth and the same was incubated at 37°C for 24 hrs. After incubation, the bacterial count was adjusted to 1×109CFU/ml by comparing the turbidity with Mac Farland Tube No. 3. The infection was given to the chicks at the age of 7th day by oral route with a single dose of 0.3 ml of nutrient broth (approximately bacterial concentration of 1×109 CFU/ml). Clinical observation and pathology The clinical Symptoms and mortality in different groups was recorded daily throughout the experimental period. The detailed necropsy examination of birds died or sacrificed on 21st and 35th day of study was performed as per standard necropsy protocol and various gross lesions were recorded. The various tissue samples were collected in 10% formal saline for histopathology during necropsy. The histopathological processing and staining (H&E staining) of various samples were performed as per standard protocol. Top RESULTS Clinical symptoms The chicks from C1 and C2 groups did not show any abnormal clinical symptoms and remained healthy throughout the experimental trial. Chicks from group T1 (E. coli infected chicks) showed symptoms from 20 hours after the infection. The prominent symptoms were dullness and depression, huddling in the corner, gasping, respiratory distress and sneezing, characteristic sniking with weakness and ruffled feathers. E. coli infected birds exhibiting clinical signs showed whitish pasty, watery, stained, diarrhoea which was intermixed with reddish colour at later stage and many birds were not inclined to eat or drink. There was pasting of vent region and feathers. The affected birds showed reduced appetite (from 1st week onwards) and less water consumption. The clinical symptoms observed in group T2 (E. coli infected + neem leaf powder) birds were dullness, occasional gasping and respiratory distress, anorexia and diarrhoea with pasting of vent. However, the birds from T2 group appeared more active than the birds from T1 group. Mortality There was no mortality observed in chicks from both control groups throughout the experimental trial. Also, the chicks were apparently healthy and active throughout the experimental period in these two groups. From group T1, 7/25 birds died (28%) during the period of experimental trail. In the first 48 hours of post infection interval there was 4% mortality, whereas 8% mortality was observed in 2nd and 3rd week of experimental period. During 4th and 5th week of study there was 4% mortality. From group T2, 3/25 birds died (12%) during the period of experimental trial. The mortality in case of group T2 birds was observed during third week. In case of group T2 birds, there was 8% and 4% mortality was observed in 3rd and 4th week, respectively while there was no mortality observed during 5th week of study. The observations of the present study revealed that addition of neem leaf powder in the diet of E. coli infected broiler birds delays as well reduces mortality rate. Gross pathology Birds from group C1 (healthy control) and group C2 (normal feed + 2 gm neem leaf powder per Kg of feed) did not show any appreciable gross pathological changes and all organs appeared normal at all intervals of study during necropsy. Hearts from T1 (E. coli infected) group birds showed marked thick yellowish fibrinous deposits on pericardium or epicardium and there was firm adhesion of pericardium to the chest wall or epicardium. At 35thday of experiment, the fibrinous pericarditis appeared more caseous in nature along with mild cardiac dilatation and paleness of myocardium. Livers from T1 group birds showed marked congestion, enlargement, appeared pale with multiple necrotic foci along with presence of thick yellow fibrinous layer over capsular surface causing thickening of capsule. The birds died or sacrificed from group T2 (E. coli infected + 2 gm neem leaf powder per kg of feed) showed less thickness or less deposition of fibrin layer over pericardium, epicardium and hepatic capsule as compared to cardiac or hepatic lesions observed in group T1 birds (Fig. 1, 2 & 3). Lungs from T1 group birds appeared congested, consolidated, pneumonic and presence of thin fibrinous layer over the surface of lungs in few birds. The air sacs appeared cloudy and thickened along with caseous deposits. Kidneys from T1 group birds showed moderate enlargement, congestion, pale discoloration along with presence of thin fibrinous layer over capsular surface. Intestine from T1 group birds showed marked congestion along with mild to moderate hemorrhagic mucoid exudates in the duodenal lumen. Spleen from T1 group sacrificed birds showed marked enlargement, hyperemia and necrotic foci. In few birds from T1 group there was distension of abdomen along with presence of straw colored fluid. Overall the severity of lesions observed in different organs of group T2 birds was comparatively lesser than group T1 birds. Histopathology The livers from group T1 & T2 birds showed marked acute fibrinous and caseous serositis/capsulitis (perihepatitis/acute fibrino-heterophilic hepatic serositis). The capsular surface of affected livers was covered with thick layer of fibrin containing predominantly heterophils. Frequently the fibrino-heterophilic mass found caseated along with presence of multiple bacterial colonies. Marked diffused hepatocellular necrosis and disruption of hepatic parenchyma/hepatic architecture was also evident. The sinusoids found dilated along with presence of fibrin thrombi. The affected livers from group T2 birds showed lesser severity of hepatic lesions than group T1 birds. Especially the thickness of fibrino- heterophilic mass/layer and hepatocellular damage (vacuolar degeneration) was found less than in group T1 birds and mild to moderate sub-epicardial hepatocellular degeneration was evident (Fig. 4,5,6). The hearts from groups T1 & T2 birds showed marked fibrinous epicarditis/pericarditis. The pericardium found markedly thickened by fibrino- heterophilic exudate (containing predominantly heterophils), caseous necrotic debris, clear spaces (edema) and congestion of blood vessels. Frequently the fibrino heterophilic mass found caseated along with presence of multiple bacterial colonies. Sub-epicardial myocarditis was also evident and characterized by disruption of the myocardium by inflammatory cells and fibrinous exudate. The other histopathological changes observed includes focal degeneration and necrosis of cardiomyocytes (loss of striations, vacuolation and fragmentation of myocytes), cardiac myocytes appeared thin and found separated by expanded interstitial spaces (oedema) (Fig. 7, 8). The kidneys from both E. coli infected groups showed marked tubular degeneration, glomerular atrophy, congestion and focal lympho-mononuclear or heterophilic infiltration. Histopathologically, the marked hemorrhagic enteritis was evident in intestines from group T1 birds while the lesions appeared more catarrhal type in case of group T2 birds. The spleen from both E. coli infected groups showed marked disruption of splenic parenchyma due to lymphoid depletion, congestion, hemorrhages, fibrinoid necrosis and perivascular heterophilic infiltration. The histopathological changes observed in lungs of both E.coli infected birds includes marked fibrino-heterophilic exudate in parabronchi (fibrino heterophilic bronchopneumonia), hemorrhages or congestion and thickening of interstitium due to heavy infiltration of inflammatory cells and fibrin. The various organs from birds of both control groups show any appreciable failed to show histopathological changes and appeared normal. Overall, the severity of histopathological lesions observed in different organs of group T2 birds was comparatively lesser than group T1 birds. Top DISCUSSION The lesions observed in present study due to experimentally induced colibacillosis were fibrinous pericarditis, fibrinous hepatitis and the inflammatory lesions were found to spread to lung, kidneys and spleen were similar to those of natural infection. The clinical symptoms and mortality observed in the group T1 were in agreement with the findings of earlier workers9,10. Dullness, depression, huddling in the corner, gasping, respiratory distress, ruffled feather, reduced feed and water intake, whitish, pasty, watery stained diarrhoea are observed in the E. coli infected birds11,12-13. Different workers recorded varied percent of mortality in chicks infected with E. coli. Higher mortality caused by E. coli infection in the present study could be due to differences in the experimental conditions, breeds of birds and the duration of study. Severity of these clinical symptoms and mortality in E. coli infected with NLP treated group birds is low due to antibacterial, antifungal, antioxidant, antiviral and hepatoprotective activity of Azadirachta |
indica4,5,14,15,16-17. Increase in cyclic adenosine monophosphate (cAMP) and cyclic guanosin monophosphate (cGMP) affects absorption of sodium as well as chloride and water balance ultimately producing diarrhoea, dehydration and death in E. coli infection18. |
The gross and histopathological lesions observed different organs in present study are consistent with several workers19,20,21,22,23-24. Accumulation of fibrinous exudates on pericardium and capsule of liver, over the surface of lungs and kidneys due to acute inflammatory reaction caused by beta hemolysin produced by E. coli resulting into marked increase in vascular permeability leads to escape and accumulation or deposition of fibrinogen in to the surrounding tissue24. The inflammatory and necrotic lesions in the liver, kidneys and heart might be due to E. coli endotoxin and vascular injury. Overall the severity of gross and histopathological lesions observed in different organs of group T2 birds was comparatively lesser than group T1 birds and these findings are in consonance with the earlier studies5,14. |
The medicinal properties of neem are reported as antibacterial, antiviral, antifungal, antioxidant and hepato-protective. Without showing any adverse effects by earlier workers and present reports supports these findings4,17. Bioassay-guided studies and photochemical analyses utilizing modern state of the art techniques such as liquid chromatography-mass spectrometry (HPLC-MS), gas chromatography-mass spectrometry (GC-MS), nuclear magnetic resonance (NMR) and infra-red spectroscopy (IR) have revealed that phytocompounds like azadirachtins, nimocinol, isomeldenin, nimbin, nimolicinol, odoratone, isoazadironolide, azadironolide, naheedin, mahmoodin are responsible for varied biological, pharmacological and toxicological properties. These constituents have been demonstrated to exhibit immunomodulatory, anti-inflammatory, anti- hyperglycaemic, antiulcer, antimalarial, antifungal, antibacterial, antiviral, antioxidant, antimutagenic and anticarcinogenic properties25,26. |
In conclusion, the oral supplementation of neem leaf powder at the dose rate 2 gm per kg of feed although could not completely prevent the birds from E. coli infection, but it induced moderate improvement in colibacillosis affected birds in terms of comparatively less severity of clinical symptoms, reduction in mortality percentage and pathological lesions. |
Top Figures | Fig. 1.: T1 group: The adhesion of thoracic organs to chest. Marked deposition of yellowish thick layer of fibrin on the surface of heart and liver
| 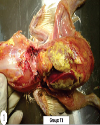 | |
| | Fig. 2.: Heart: T1 group-marked thickening of pericardium due to deposition of yellowish fibrin deposits. Reduced severity of lesions in heart from group T2
| 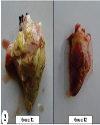 | |
| | Fig. 3.: Group Tl-Marked deposition of yellowish thick fibrinous layer on capsular surface of liver. Group T2: Thin whitish fibrin layer and reduced severity of lesions
| 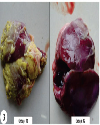 | |
| | Fig. 4.: Liver: (T1 group): Fibrino-heterophilic caseous hepatic serositis. H&E ×40
|  | |
| | Fig. 5.: Liver: T1 group: Fibrino-heterophilic caseous hepatic serositis. Presence of numerous bacterial colonies in caseous mass (yellow circles). H&E ×200
| 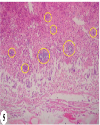 | |
| | Fig. 6.: Liver: (T2 group): Fibrino-heterophilic hepatic serositis: Less thickness of fibrino heterophilic mass present over capsular surface of liver. H&E ×100
| 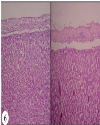 | |
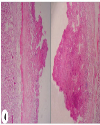
| | Fig. 7.: Heart: T1 group: Fibrino heterophilic epicarditis/pericarditis: Fibrino heterophilic caseous mass present over epicardial surface of heart. H&E ×100
| 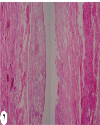 | |
| | Fig. 8.: Heart: T2 group: Fibrino-heterophilic epicarditis/pericarditis: Less thickness of fibrino heterophilic mass present over epicardial surface of heart. H&E ×100.
| 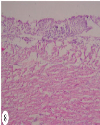 | |
|
|